45 regulations require that product labels on containers
California Code of Regulations, Title 8, Section 5193 ... 5. Red bags or red containers may be substituted for labels except for sharp containers or regulated waste red bags. Bags used to contain regulated waste shall be color-coded red and shall be labeled in accordance with subsection (g)(1)(A)2. Labels on red bags or red containers do not need to be color-coded in accordance with subsection (g)(1)(A)3. 1910.1030 - Bloodborne pathogens. | Occupational Safety and ... Red bags or red containers may be substituted for labels. 1910.1030(g)(1)(i)(F) Containers of blood, blood components, or blood products that are labeled as to their contents and have been released for transfusion or other clinical use are exempted from the labeling requirements of paragraph (g).
Federal Register :: National Bioengineered Food Disclosure ... Dec 21, 2018 · These terms are critical in determining what foods require a BE disclosure. B. Food Subject to Disclosure. Whether a food is subject to the labeling requirements of the amended Act, depends as a preliminary matter on whether the product at issue is a food.

Regulations require that product labels on containers
Packaging and labeling - Wikipedia The purposes of packaging and package labels. Packaging and package labeling have several objectives. Physical protection – The objects enclosed in the package may require protection from, among other things, mechanical shock, vibration, electrostatic discharge, compression, temperature, etc. Canada Gazette, Part 2, Volume 156, Number 14: Regulations ... Jul 06, 2022 · In this analysis, businesses will either continue with existing labelling configurations, footnote 59 or change to use a different labelling configuration, such as peel-back labels, or choose to adopt outer boxes, inserts or leaflets. footnote 60 No product discontinuation is assumed because these amendments do not require any product to stop ... Cosmetics Labeling Guide | FDA Regulations require that "[the label of a cosmetic product shall bear a warning statement whenever necessary or appropriate to prevent a health hazard that may be associated with the product" [21 ...
Regulations require that product labels on containers. California Code of Regulations, Title 8, Section 5194. Hazard ... Sep 28, 2018 · (B) Any food, food additive, color additive, drug, cosmetic, or medical or veterinary device, including materials intended for use as ingredients in such products (e.g., flavors and fragrances), as such terms are defined in the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 301 et seq.) and regulations issued under that Act, when they are subject to the labeling requirements of that Act and ... Cosmetics Labeling Guide | FDA Regulations require that "[the label of a cosmetic product shall bear a warning statement whenever necessary or appropriate to prevent a health hazard that may be associated with the product" [21 ... Canada Gazette, Part 2, Volume 156, Number 14: Regulations ... Jul 06, 2022 · In this analysis, businesses will either continue with existing labelling configurations, footnote 59 or change to use a different labelling configuration, such as peel-back labels, or choose to adopt outer boxes, inserts or leaflets. footnote 60 No product discontinuation is assumed because these amendments do not require any product to stop ... Packaging and labeling - Wikipedia The purposes of packaging and package labels. Packaging and package labeling have several objectives. Physical protection – The objects enclosed in the package may require protection from, among other things, mechanical shock, vibration, electrostatic discharge, compression, temperature, etc.











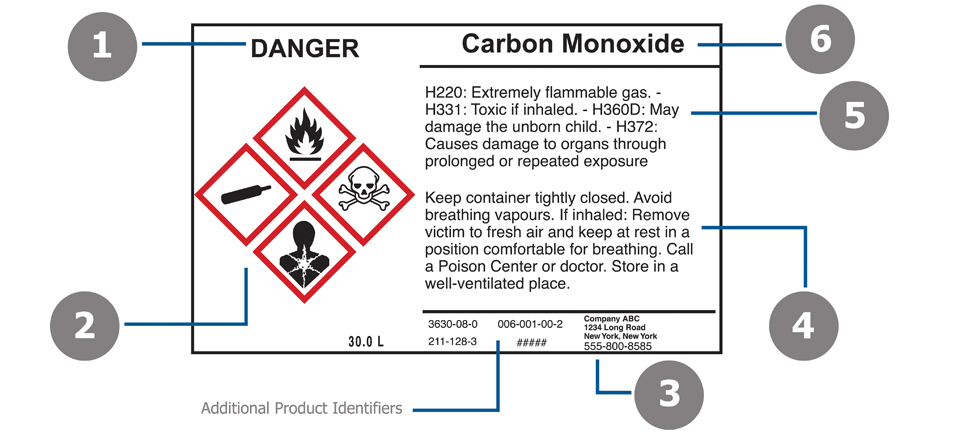




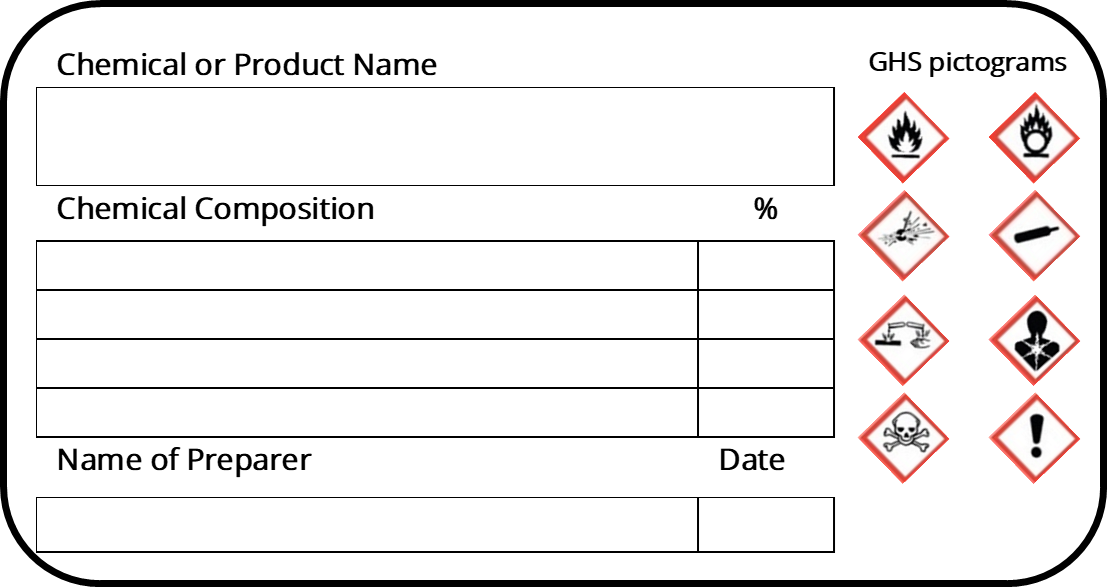
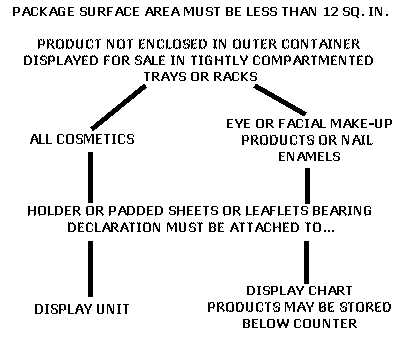




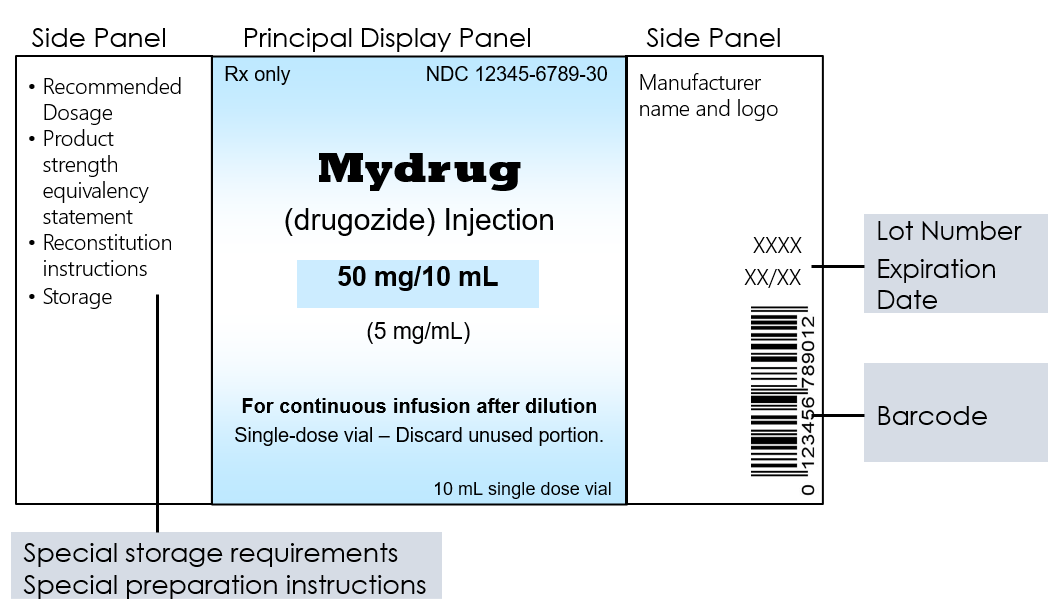





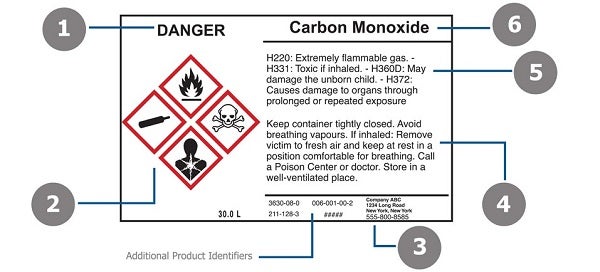




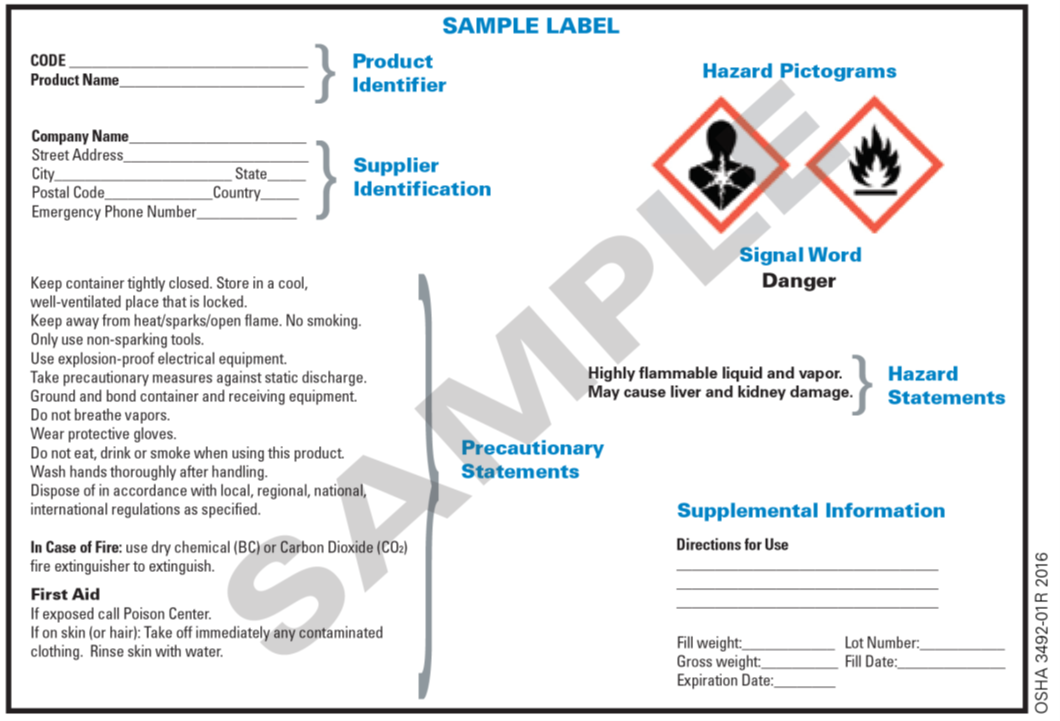

Post a Comment for "45 regulations require that product labels on containers"