38 fda health claims on food labels
FDA perspectives on health claims for food labels - PubMed The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA). This law established mandatory nutrition labeling for most foods and placed restrictions on the use of food label claims charact … How to Start a Food Business | FDA Food Businesses Subject to FDA Regulation. FDA regulates all foods and food ingredients introduced into or offered for sale in interstate commerce, with the exception of meat, poultry, and certain ...
› organic-food-labelsOrganic on Food Labels | FDA Oct 12, 2022 · The FDA does not regulate the use of the term “organic” on food labels, including dietary supplements. The National Organic Program (NOP) is the federal regulatory framework governing ...

Fda health claims on food labels
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ... Food/Dietary Supplement Guidance and Regulatory Information Sep 30, 2022 · Acidified and Low-Acid Canned Foods: Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food Process Filing Forms) to FDA ... Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Fda health claims on food labels. › food › dietary-supplements-guidanceDietary Supplement Labeling Guide: Chapter VI. Claims | FDA U.S. Food and Drug Administration, Guidance for Industry, Interim Procedures for Qualified Health Claims in the Labeling of Conventional Human Food and Human Dietary Supplements July 2003. FDA Proposes New 'Healthy' Claim on Food Labels FDA Proposes New 'Healthy' Claim on Food Labels. Sept. 28, 2022. Its food group-based approach continues prohibitions but allows salmon and nuts to be considered healthy. The FDA today (Sept. 28) issued a proposed rule to update the definition of the "healthy" claim on food & beverage packaging. Interested parties have about three ... Fda Health Claims On Food Labels FDA Proposes to Update Definition for "Healthy" Claim on … Health (7 days ago) September 28, 2022. The U.S. Food and Drug Administration today issued a proposed rule to update the definition of the nutrient content claim "healthy.". 5 Understanding Food Labels and Health Claims - Maricopa To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease." A qualified claim has supportive evidence, which is NOT ...
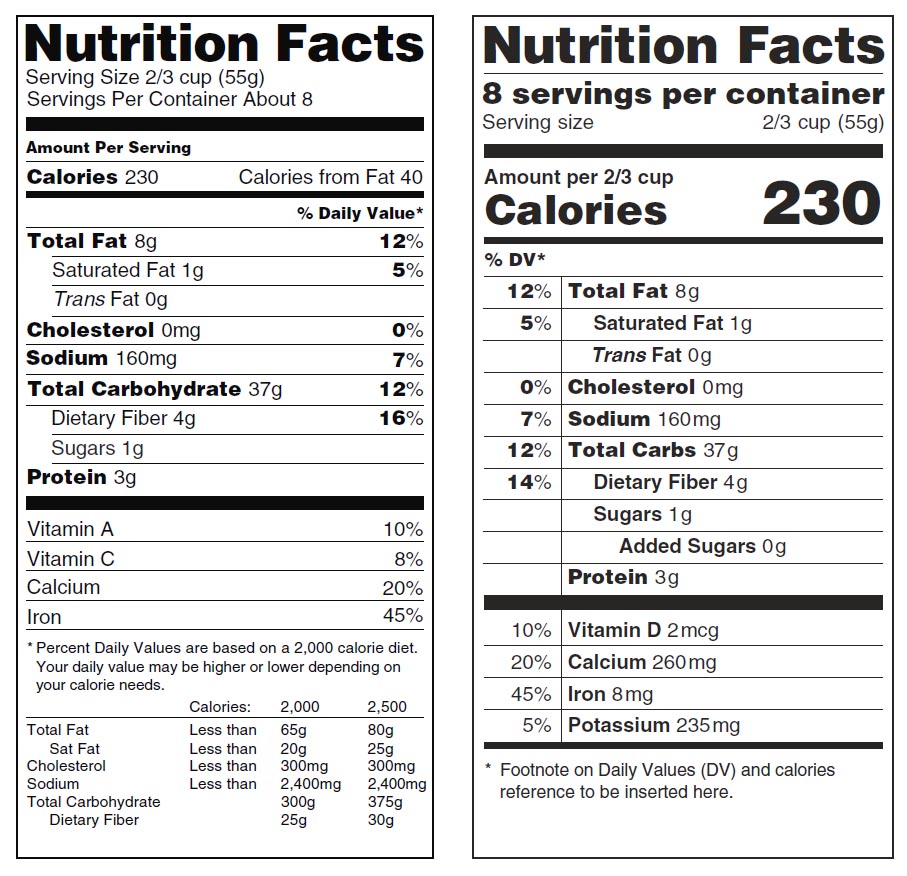
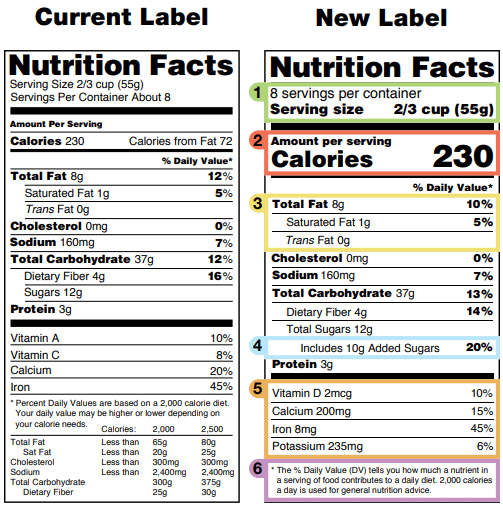
Fda Health Claims Food The FTC regulates all forms of advertising. FDA regulates the content and form of labels for food, cosmetics, dietary supplements, devices and drugs.Labels are simply a special form of advertising, so both FTC and FDA regulate them.FDA reviews labels for compliance and to see that you meet the basic requirements to be in the market. › food › food-industryHow to Start a Food Business | FDA - U.S. Food and Drug ... Food Businesses Subject to FDA Regulation. FDA regulates all foods and food ingredients introduced into or offered for sale in interstate commerce, with the exception of meat, poultry, and certain ... › food › guidance-regulation-food-andFood/Dietary Supplement Guidance and Regulatory Information Sep 30, 2022 · Acidified and Low-Acid Canned Foods: Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food Process Filing Forms) to FDA ... Changes to the Nutrition Facts Label | FDA - U.S. Food and Drug ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...
› food › food-labeling-nutritionLabel Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA - U.S. Food ... U.S. Food and Drug Administration, Guidance for Industry, Interim Procedures for Qualified Health Claims in the Labeling of Conventional Human Food and Human Dietary Supplements July 2003. Everything you need to know about Health Claims on Food Labels A nutrient content claim must be true and accurate just like health claims, it is a statement about the amount of a nutrient found in a food. Usually placed on the front of the food label, the nutrient claim provides a quick comparison between similar products. Some examples of nutrient claims are "low sodium", "high in fiber", " fewer calories", › regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims | FDA On January 6, 2000, the Food and Drug Administration (FDA) published a final rule in the Federal Register defining the types of statements that may be used on the label and in the labeling of ...
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food...
FDA Regulation of Cannabis and Cannabis-Derived Products: Q In addition, under 21 CFR 530.20(b)(2), if scientific information on the human food safety aspect of the use of the approved human drug in food-producing animals is not available, the veterinarian ...
› food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Food Allergies | FDA - U.S. Food and Drug Administration Jun 23, 2022 · People with food allergies should read labels and avoid the foods they are allergic to. The law requires that food labels identify the food source of all major food allergens used to make the food.
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." It is critical that companies familiarize themselves with ...
Small Entity Compliance Guide on Structure/Function Claims | FDA On January 6, 2000, the Food and Drug Administration (FDA) published a final rule in the Federal Register defining the types of statements that may be used on the label and in the labeling of ...
FDA proposes updates to 'healthy' claim on food packages | CNN In order to be labeled with the "healthy" claim, products would need to: Contain a certain, meaningful amount of food from at least one of the food groups or subgroups - such as fruits,...
Organic on Food Labels | FDA Oct 12, 2022 · The FDA does not regulate the use of the term “organic” on food labels, including dietary supplements. The National Organic Program (NOP) is the federal regulatory framework governing ...
Label Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Food/Dietary Supplement Guidance and Regulatory Information Sep 30, 2022 · Acidified and Low-Acid Canned Foods: Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food Process Filing Forms) to FDA ...
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...






























Post a Comment for "38 fda health claims on food labels"