42 phase labels in chemical equations
A thermochemical equation is a balanced chemical reaction equation ... An equation which shows both mass and heat relationships between products and reactants is called a thermochemical equation. The following four reactions are examples of thermochemical equations. The first two are exothermic and the last two are endothermic reactions. 2 H 2 (g) + O 2 (g) ----> 2 H 2 O(l) DH = -571.6 kJ Chemical Reactions and Equations - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). ... According to the solubility rules, Ca 3 (PO 4) 2 is insoluble, so it has an (s) phase label. To balance this equation, we need two phosphate ions and three calcium ions; we end ...
Chapter 6 General Chemistry Flashcards - Quizlet The chemical equation for a reaction (including phase labels) in which the equation is given a molar interpretation, and the enthalpy of reaction for these molar amounts is written directly after the equation ... When a chemical equation is reversed, the value of ΔH is reversed in sign. calorimeter. A device used to measure the heat absorbed ...

Phase labels in chemical equations
PDF Experiment 6 Chemical Reactions - Anoka-Ramsey Community College can fill in the phase labels for the products of the reaction as follows: Pb(NO 3) 2 (aq) + Na 2 SO 4 (aq) → PbSO 4 (s) + NaNO 3 (aq) After determining the phases of the products, the equation must be balanced to satisfy the Law of Conservation of Mass. The result is the following balanced "molecular" chemical equation: Pb(NO 3) 2 (aq ... Chemical Formula Labels Teaching Resources | Teachers Pay Teachers Use this digital interactive notebook to teach your students about chemical formulas and chemical reactions.The journal introduces and walks students through how to count the atoms.Then they break down an equation with drag and drop labels.The students end with vocabulary and scenarios. Subjects: Earth Sciences, General Science, Physical Science Neutralization Reactions - GitHub Pages Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. For example, the chemical reaction between HCl(aq) and Fe(OH) 3 (s) still proceeds according to the equation 3HCl(aq) + Fe(OH) 3 (s) → 3H 2 O(ℓ) + FeCl 3 (aq) even though Fe(OH) 3 is not soluble. When one realizes that Fe(OH) 3 (s) is a component of rust, this explains ...
Phase labels in chemical equations. State symbols and phase changes - StudyPug Building on chemical equations Chemical and physical changes. Chemical phases and state symbols. Other key phase/state language. Phase changes. Examples Lessons Recall the different states of matter from descriptions of chemicals. Write "solid", "liquid", "gas", or "aqueous", next to each term below to show which state it is describing. Powder Solved D. WRITE COMBUSTION EQUATIONS 1. Write a balanced | Chegg.com Write a balanced chemical equation describing the combustion of heptane (C7H16). Reference the Background Information section to determine the other reactant and the products. Make sure to include phase labels on all reactants and products. 2. Write a balanced chemical equation describing the combustion of wax (C21H44). Chemical equations Flashcards | Quizlet 2nd Semester Chemistry Vocabulary. 24 terms. lporter1649. Chemical Reaction Test Study Quizlet. 45 terms. donellevans. OTHER SETS BY THIS CREATOR. safety and risk. 19 terms. Complete and balance each of the following molecular equations ... Complete and balance each of the following molecular equations, including phase labels, if a reaction occurs. Then write the net ionic equation. If no reaction occurs, write NR after the arrow. H2CO3 (aq)+Sr (OH)2 (aq) -> this is what I put but I think its wrong H2CO3 (aq)+Sr (OH)2 (aq) -> SrO (aq)+CO2 (g)+H2O (l)
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways How do you Write a Chemical Equation? - A Plus Topper 1. To balance Y on both sides, multiply RHS by 2, i.e., X + Y 2 2XY. Now, the number of atoms of Y is balanced but not the number of atoms of X. Therefore, multiply X on the LHS by 2. Thus, the equation becomes. 2X + Y 2 2XY. This is a balanced equation as the number of atoms of X and Y on both sides is equal. The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Conventions for Writing Chemical Equations - Arrows, Phases ... presents: Conventions for Writing Chemical Equations including Arrows, Phases, Coefficients, Reactants, Products and moreTired...
Solved 1. Chemical equations can also be used to represent | Chegg.com Write a chemical reaction for the boiling of water, including the proper phase labels. 2. Chemical equations can also be used to represent physical processos. Write a chemical reaction for the freezing of water, including the proper phase labels 3. Explain why 4Na (s) + 2C12 (g) - NaCl (s) should not be considered a proper chemical equation. 4. 3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g) Solved write a balanced chemical equation including phase | Chegg.com This problem has been solved! See the answer write a balanced chemical equation including phase labels for the reaction between aqueous copper (II) nitrate and aqueous sodium hydroxide Best Answer 100% (1 rating) 1] write equation in words first, you have all the products given except there is also water in the first. 2] rewrite, subs … Chemical Equations - GitHub Pages If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H 2 (g) + O 2 (g) → H 2 O(ℓ) Note. ... To make this chemical equation conform to the law of conservation of matter, we must revise the amounts of the reactants and the products as necessary to get the same ...
Solved Phase labels never appear in balanced chemical | Chegg.com Answer to Solved Phase labels never appear in balanced chemical
4.1 Writing and Balancing Chemical Equations - Chemistry Write a balanced equation for the reaction of molecular nitrogen (N 2) and oxygen (O 2) to form dinitrogen pentoxide. Solution. First, write the unbalanced equation. N2 +O2 N2O5 (unbalanced) N 2 + O 2 N 2 O 5 ( unbalanced) Next, count the number of each type of atom present in the unbalanced equation. Element.
PDF Chapter 8 chemical equations and reactions test answer key Write a chemical reaction to freezing water, including the appropriate phase labels. Explain why 4NA (s) + Ã, 2cl2 (g) Ã, Ã, 4nacl (s) should not be considered a real chemical equation. Ã, explain why H2 (G) + 1 / 2O2 (G) A at H2O (a) One should not be considered a real chemical equation.
5.7 End-of-Chapter Material - Introductory Chemistry - NSCC Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation.
Phase Transitions: Melting, Boiling, and Subliming Chemical equations can be used to represent a phase change. In such cases, it is crucial to use phase labels on the substances. For example, the chemical equation for the melting of ice to make liquid water is as follows: H 2 O(s) → H 2 O(ℓ) No chemical change is taking place; however, a physical change is taking place. Heating Curves
End-of-Chapter Material - GitHub Pages Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
Write a balanced thermochemical equation with phase labels for the ... 2. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation. (3 pts) N2 (g)+ 3 H2 (g)→ 2 NH3 (g) (ΔH = −92.22 kJ) 3. What is the theoretical yield of ammonia (in grams) if 15.85 grams of nitrogen gas and 11.40 grams of hydrogen gas are allowed to react? (11 pts) Nitrogen ...
What are Chemical Equations? Detailed Explanation, Examples The reactants and the products (for which the chemical formulae are written in chemical equations) can be separated by one of the following four symbols. In order to describe a net forward reaction, the symbol '→' is used. In order to describe a state of chemical equilibrium, the symbol '⇌' is used.



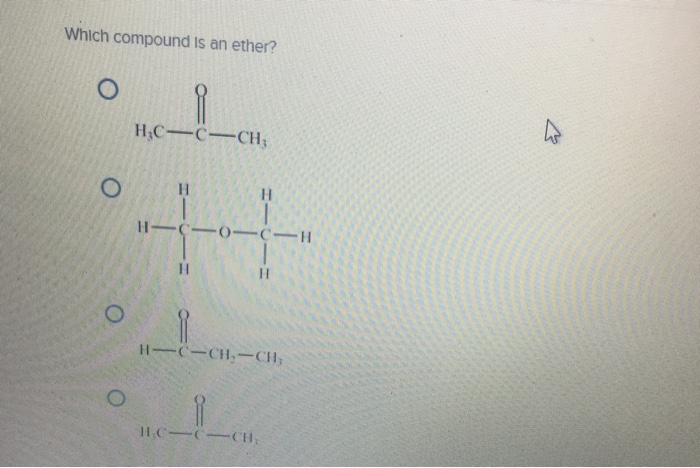
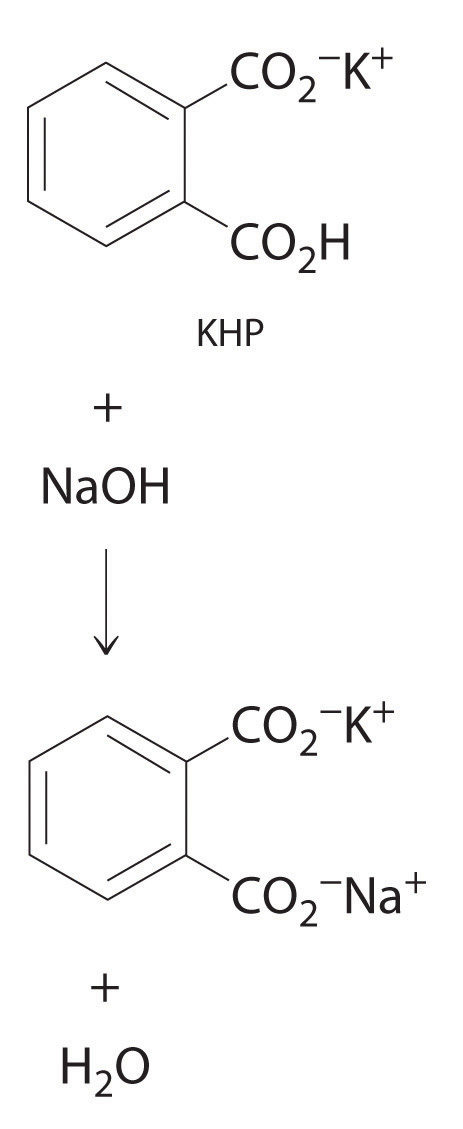
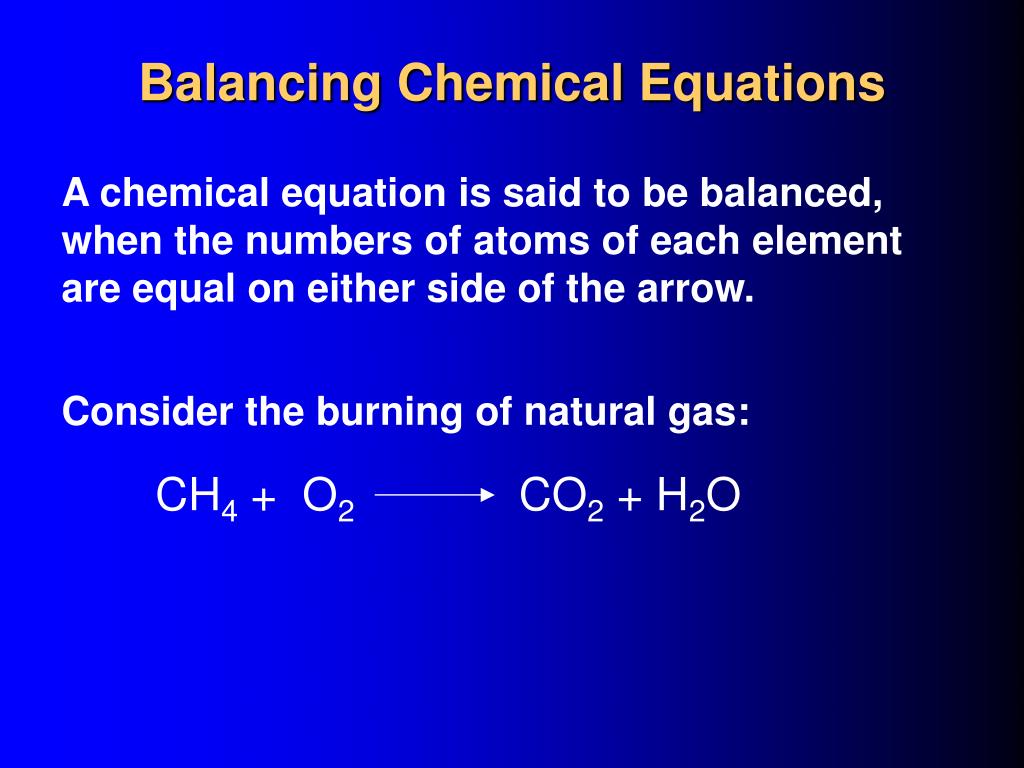
Post a Comment for "42 phase labels in chemical equations"